Travel with peace of mind: opt for a private driver at the airport
Air travel can often be stressful and tiring, especially when it comes to reaching a busy airport. Between traffic jams, parking problems and the rush to arrive on time, it…
SEO copywriting: why is the choice of copywriter crucial?
Writing content for the web has become a major challenge for any business or website that wants to stand out online. Among the many writing techniques, SEO (Search Engine Optimization)…
The elegant styles and designs of silk sleeping masks
You’ve no doubt heard about the health benefits of sleep. But did you know that the quality of your sleep can be influenced by the environment in which you sleep?…
Book your airport limousine transfer
If you’re looking for the most luxurious and convenient way to get to the airport, book your trip in a limousine. It’s a popular way to travel in style and…
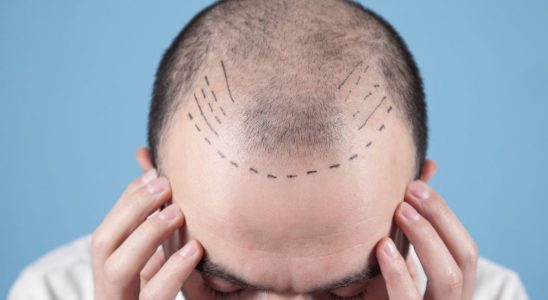
Pre-transplant preparations for hair grafts
A revolutionary technique to achieve complete coverage of the bald area is the hair transplant. The technique has been significantly improved in recent years and pre-transplant preparations can play a…
Good plans to rent a car at low cost
Renting a car can be very expensive if you are not well informed. Fortunately, it is always possible to find good deals on renting a vehicle at a low price.…
The importance of complex passwords
Passwords are one of the most important keys to securing an online account. Yet many people use weak and insecure passwords. In this article, we will discuss the importance of…
Seamstresses’ secrets to give volume to a tulle dress
The wedding dress is the essential element of any wedding ceremony. Among the many options, tulle offers a touch of femininity and softness, underlined by a nicely puffed skirt. But…
Best Practices for EV Home Charger Installation
The installation of an Electric Vehicle (EV) Home Charger is an important step for many EV owners. Ensuring the charger is properly installed and maintained is essential for safe and…
10 reasons to visit Bonifacio by boat
With its port and its white houses overlooking the Mediterranean Sea, Bonifacio is a city not to be missed when visiting Corsica. But what to do in Bonifacio? No need…